"Panda blood" refers to the Rh-negative blood type in China, which, as the phrase suggests, is "as rare as pandas". It is estimated that about 3 out of 1000 Chinese people have the Rh-negative blood type.
Due to its extreme rarity, Rh-negative blood is constantly in short supply. Whether this life-saving blood can be available in a timely manner plays a critical role in the life of the patient with the Rh-negative blood type.
To address this challenge, the research team led by TANG Ruikang, a professor from the Department of Chemistry, and WANG Ben, an associate professor from the Second Affiliated Hospital of the Zhejiang University School of Medicine, successfully engineered universal "panda blood." They developed an alternative approach to sheltering the epitopes on RhD-positive RBCs using a surface-anchored framework and achieved the fabrication and transfusion of this universal "panda blood". These findings are published in the March 20 issue of Science Advances.
The pioneering studies of Karl Landsteiner in the 19th century provided a solid foundation for our current understanding of blood types. The major human alloantigen system involves the ABO, Rhesus (Rh), MNS, Lutheran, and Kell blood groups. The Rh blood group system is a clinically notable human blood group polymorphism in alloimmunization and includes more than 50 different serologic specificities, which makes this system particularly paramount and challenging for biomedical scientists and clinicians. Within the Rh blood group, the D antigen is the most immunogenic and clinically vital epitope. As a rare blood type, there is a dire deficiency in the bank of RhD-negative blood for transfusion, especially in emergencies. Exposure of RhD-negative individuals to RhD-positive blood may cause serious immune responses, including hemolysis, which is life threatening.
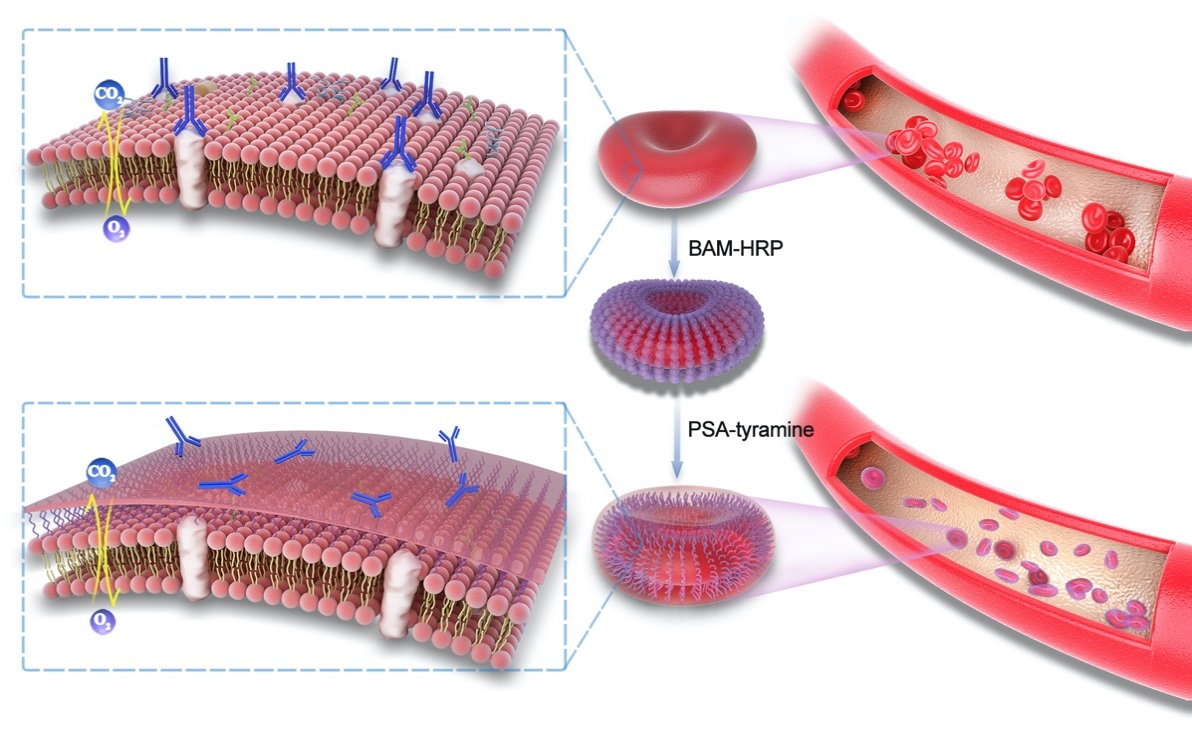
Schematic illustration of the procedure for RBC surface engineering
Is there any "once-and-for-all" solution to this formidable challenge? The research team led by TANG Ruikang and WANG Ben developed a novel technique to engineer cell surfaces for the production of RhD-negative RBCs using a rationally designed three-dimensional (3D) crosslinking framework of polysialic acid (PSA)-tyramine hydrogel, which has a flexible texture to ensure cell membrane stability. This technique for the anchored framework can balance the modified RBC membrane fluidity and RhD antigen shielding and achieve the successful transfusion of the engineered RhD-positive RBCs to RhD-negative recipients without immunogenicity.
This universal "panda blood" was already tested in a mouse model and these results indicated that surface engineering leads to negligible complement activation and cytokine response and presents good biocompatibility. Furthermore, the systematic biosafety of engineered RBCs was investigated in a rabbit model, which indicated that surface modifications can not only bypass anti-RhD immunity but also promote favorable biocompatibility. This study, therefore, holds great promises for clinical translation.
This study demonstrates a biocompatible membrane-anchoring self-assembly method for cell surface engineering. This approach may promote immunological applications by sheltering antigens of tissues and cells at the single-cell level. Researchers believe that the application of cell surface engineering will provide an alternative to preventing rejection reactions with control over cellular functions through material transformation.
In addition to our follow-up research into universal red cells, the compatibility issue in platelet transfusion is more troublesome, WANG Ben said. "In the not-too-distant future, there may be more chemical-biological ways to convert cells and endow them with more functions, opening up the possibility of their applications in medicine," WANG Ben added.






