We often think about diseases in terms of the symptoms they present. A cold might give you the sniffles or even GI distress, while malaria can give you fever, chills, or nausea, for example. These symptoms, although not pleasant, are referred to as positive symptoms. But we don't often think of the things that diseases can take from you.
The positive symptoms of schizophrenia-those that are not generally present in people without the disease-include delusions, hallucinations, disorganized thinking, and abnormal body movements, are treated by current therapies, including both typical and atypical antipsychotics. Negative symptoms, however-behaviors that are missing or are underdeveloped in people with the disease, such as lack of emotional expression or social withdrawal-are not treatable with current therapies, which often leads to patient non-adherence.

Recent research from the Warren Center for Neuroscience Drug Discovery, published in the Journal of Medicinal Chemistry, resulted in the proposal of a novel target and mechanism for improving cognition-a negative symptom-while also treating positive symptoms of the disease.

The research was spearheaded by the groups of Craig Lindsley, University Distinguished Professor of Pharmacology and Biochemistry and executive director of the WCNDD, and collaborators at Boehringer Ingelheim, Daniel Ursu and Henning Priepke.
The WCNDD is a leader in the field of allosteric modulation of metabotropic glutamate receptors. Glutamate, an amino acid that serves as an excitatory neurotransmitter, binds to these mGlu receptors. The WCNDD approach uses positive allosteric modulators to target receptors at sites outside their main ligand-binding pockets (orthosteric sites). In a recent paper, WCNDD investigators explored the feasibility of developing novel PAMs for mGlu1.
Developing PAMs is more challenging than developing agonists that bind at orthosteric sites but allows for achieving subtype selectivity. In the case of mGlu1, the researchers posited that this allosteric modulation strategy would yield a compound with the subtype selectivity needed to study the in vivo effects of mGlu1 without significantly interacting with any of the other mGlu2-mGlu8 subtypes.
To identify potential novel mGlu1 PAMs, the researchers conducted a high-throughput screen and performed chemical lead optimization efforts that led to the discovery of VU6024578/BI02982816. Using VU6024578/BI02982816, the researchers performed proof-of-concept studies in rodent preclinical models of psychosis and cognition, psychosis being a positive symptom of schizophrenia and deficits in cognition being a negative symptom.
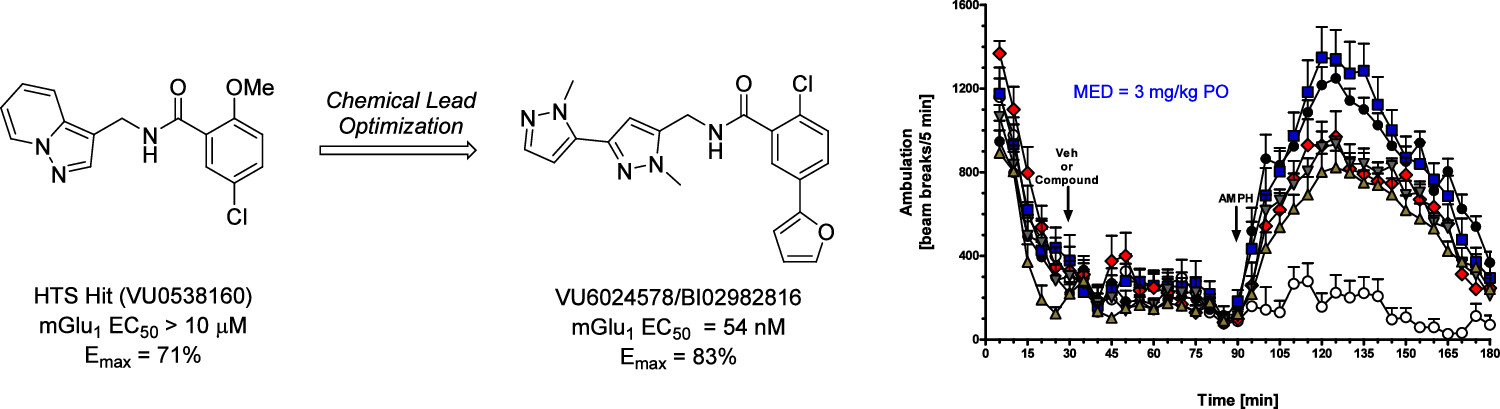 10 µM. Emax = 71%." An arrow labeled "chemical lead optimization" flows to the right into a related, 3-ringed compound labeled "VU6024578/BI02982816. mGlu1 EC50 = 54 nM. Emax = 83%." The graph on the right has six lines with data points marked in different shapes and sizes. The axes are time (x-axis, min) and ambulation (y-axis, beam breaks/5 min). A key is not provided but can be found in Figure 7 of the paper." width="1500" height="409" srcset="https://cdn.vanderbilt.edu/t2-main/medschool-prd/wp-content/uploads/sites/101/2025/01/2024-JMC-graphical-abstract.jpeg 1500w, https://cdn.vanderbilt.edu/t2-main/medschool-prd/wp-content/uploads/sites/101/2025/01/2024-JMC-graphical-abstract-300x82.jpeg 300w, https://cdn.vanderbilt.edu/t2-main/medschool-prd/wp-content/uploads/sites/101/2025/01/2024-JMC-graphical-abstract-1024x279.jpeg 1024w, https://cdn.vanderbilt.edu/t2-main/medschool-prd/wp-content/uploads/sites/101/2025/01/2024-JMC-graphical-abstract-768x209.jpeg 768w" sizes="(max-width: 1500px) 100vw, 1500px">
10 µM. Emax = 71%." An arrow labeled "chemical lead optimization" flows to the right into a related, 3-ringed compound labeled "VU6024578/BI02982816. mGlu1 EC50 = 54 nM. Emax = 83%." The graph on the right has six lines with data points marked in different shapes and sizes. The axes are time (x-axis, min) and ambulation (y-axis, beam breaks/5 min). A key is not provided but can be found in Figure 7 of the paper." width="1500" height="409" srcset="https://cdn.vanderbilt.edu/t2-main/medschool-prd/wp-content/uploads/sites/101/2025/01/2024-JMC-graphical-abstract.jpeg 1500w, https://cdn.vanderbilt.edu/t2-main/medschool-prd/wp-content/uploads/sites/101/2025/01/2024-JMC-graphical-abstract-300x82.jpeg 300w, https://cdn.vanderbilt.edu/t2-main/medschool-prd/wp-content/uploads/sites/101/2025/01/2024-JMC-graphical-abstract-1024x279.jpeg 1024w, https://cdn.vanderbilt.edu/t2-main/medschool-prd/wp-content/uploads/sites/101/2025/01/2024-JMC-graphical-abstract-768x209.jpeg 768w" sizes="(max-width: 1500px) 100vw, 1500px">First, the researchers looked at the effects of VU6024578/BI02982816 on rats and mice with amphetamine-induced hyperlocomotion, a model for psychosis. The authors investigated whether the compound could reverse the hyperlocomotion and found that the reversal was dose dependent.
Then, for the cognition studies, the researchers tested rats' reaction to novel objects. Typically, rats spend more time exploring a novel object compared to a familiar object, but pretreatment with a compound called MK-801 decreases the amount of time the rats spend exploring a novel object. When rats pretreated with MK-801 are then treated with VU6024578/BI02982816, the authors observed a reversal in the exploration deficits and saw a restoration in the time spent exploring the novel object.
Following the rodent experiments, the researchers investigated the pharmacokinetics of VU6024578/BI02982816 in dogs. Unfortunately, there were unanticipated adverse events (mainly salivation and rigidity) when the dogs were exposed to the compound, but the AEs subsided within 24 hours. As rats tolerated a much higher concentration of VU6024578/BI02982816 without the presence of AEs, further experiments are required to determine their mechanistic source.
Although the observed AEs in higher species unfortunately precluded the development of this compound, this best-in-class, rodent-selective in vivo tool compound will allow deeper interrogation of mGlu1, which can lead to a better understanding of the pharmacological implications and mechanistic pharmacology of this receptor.
Research building on these results could lead to the identification and development of new compounds devoid of AEs in higher species. If achieved, this would provide a novel therapeutic target and mechanism for treating the cognitive deficits and other positive symptoms of schizophrenia.
This work was the result of a collaborative effort amongst the talented teams of scientists at the WCNDD and BI, led by Lindsley and Ursu, respectively. Medicinal chemistry was led by Bruce Melancon and Priepke. Molecular pharmacology was led by Colleen Niswender and Hyekyung Plumley. Drug metabolism and pharmacokinetics was led by Olivier Boutaud and Annie Blobaum. Behavioral pharmacology was led by Jerri Rook.
Go deeper
The paper "Discovery of VU6024578/BI02982816: An mGlu1 Positive Allosteric Modulator with Efficacy in Preclinical Antipsychotic and Cognition Models" was published in the Journal of Medicinal Chemistry in December 2024.
Funding
This research used funds from a sponsored research agreement with Boehringer Ingelheim and from the Warren Center for Neuroscience Drug Discovery, which is supported by William K. Warren, Jr. and the William K. Warren Foundation.






