We're used to thinking of the immune system as a separate entity, almost a distinct organ, but the truth is much more complicated. Breakthroughs in recent years - some resulting from research performed in Prof. Rotem Sorek's lab at the Weizmann Institute of Science's Molecular Genetics Department - have shown that individual bacterial cells possess their own autonomous, innate immune system that can identify, locate and deal with intruders. In a new paper recently published, Sorek's team - along with collaborators from Harvard Medical School and the Dana-Farber Cancer Institute - have revealed both the way in which viruses overcome a cell's immune system and the chemical composition of a mysterious molecule that's inherent to the process.

The viruses that cause bacterial cells to raise their defense shields are called phages. These viruses' modus operandi is to inject their DNA into a bacterium, manipulating the cell to replicate the phage dozens of times. At that point, the newly born phages kill the bacterium, break out and go hunting for other nearby bacterial cells. However, the bacteria are not defenseless, employing their autonomous immune system to combat this threat.
Previous research at Sorek's lab had shown that an immune protein segment called TIR is the one in charge of identifying a phage invasion and that once a phage is detected, the TIR produces a mysterious signal molecule that triggers the immune response. The TIR segment was initially discovered in the immune systems of plants and animals, but Sorek's group was able to demonstrate that a similar mechanism exists in bacteria. Still, the mystery signal molecule remained undetected.
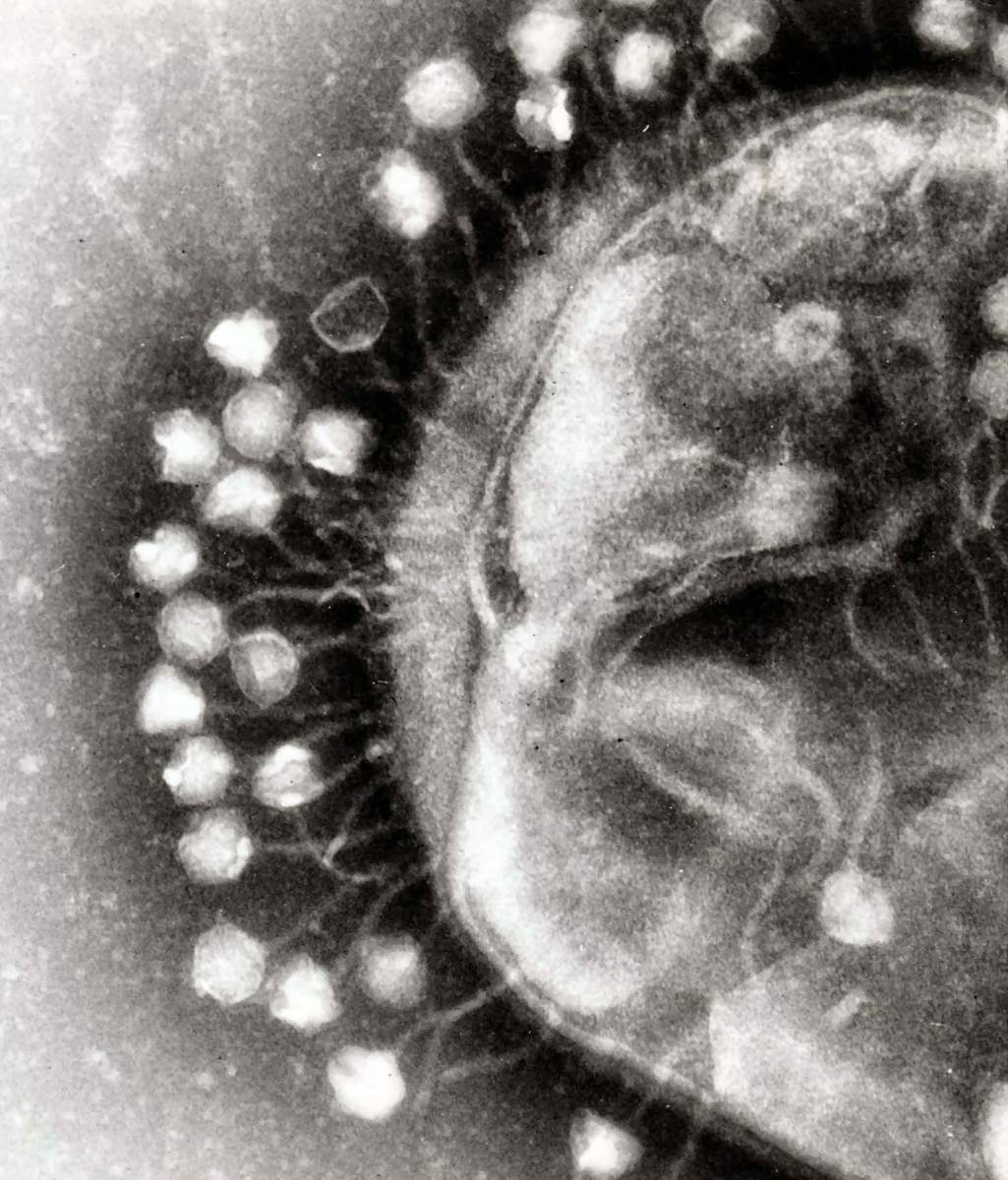
This time around, Sorek's team found how phages can overcome TIR immunity. When studying a group of very similar phages, they were surprised to discover that while TIR immunity did provide protection against some of them, others proved victorious and managed to kill the bacteria. Looking into the victorious phages, the team found that they contain a special gene, one encoding a protein that neutralizes TIR immunity, thus allowing the phage to gain the upper hand.
""We will not be surprised if viruses that infect our body use the exact same mechanism as the one we found in phages"
When the scientists examined the protein, now dubbed Tad1, they found that it captures the signaling molecule immediately after it is produced by the TIR protein. "It was as if the protein quickly swallowed the molecule, not letting the immune system get even a glimpse of it," Sorek says. "This kind of immune evasion mechanism was never seen in any known virus."
The group then realized that if the molecule is locked inside the phage protein, they might be able to "see" it by looking within the structure of the protein. Together with their collaborators from Harvard, Prof. Philip Kranzusch and Allen Lu, the team was able, via crystallography, to determine the spatial structure and chemical composition of the molecule. "We have sought this mysterious immune molecule for several years now," muses Sorek. "Ironically, we couldn't have found it without an assist from the phage."
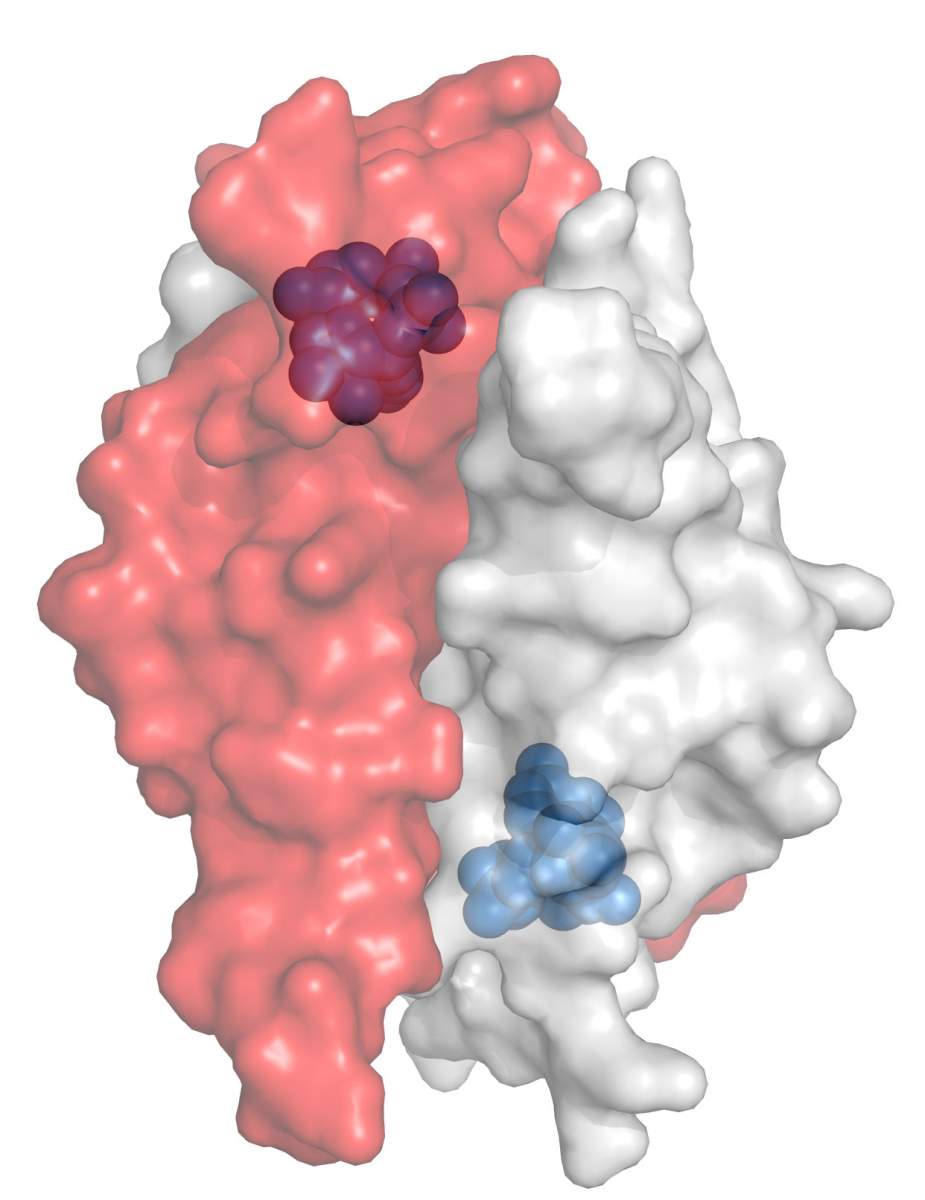
"We discovered a new way in which viruses can deactivate immune systems that rely on signaling molecules," says Sorek. "These immune systems aren't exclusive to bacteria - they exist in the cells of plants and human beings." Understanding how phages are able to adapt and evolve could help us fare better against the bacterial immune system by identifying the same mechanisms in the cellular structure of the viruses that trouble us. "We will not be surprised if viruses that infect our body use the exact same mechanism as the Tad1 we found in phages," Sorek says. If this is the case, then it could have direct consequences on our ability to protect ourselves from viruses that are scheming to outsmart our immune system.
Science Numbers
A phage-infected bacterium can produce approximately 100 new phages in less than 1 hour.
Study participants also included Dr. Azita Leavitt, Erez Yirmiya, Dr. Gil Amitai, Jeremy Garb and Dr. Ehud Herbst of Prof. Sorek's lab at Weizmann; Drs. Benjamin R. Morehouse, Samuel J. Hobbs and Sadie P. Antine of Harvard Medical School and the Dana-Farber Cancer Institute; and Dr. Zhen-Yu J. Sun of the Dana-Farber Cancer Institute.
Prof. Rotem Sorek is the head of the Knell Family Center for Microbiology. Prof. Sorek's research is supported by the Willner Family Leadership Institute for the Weizmann Institute of Science; the Dr. Barry Sherman Institute for Medicinal Chemistry; the Sagol Weizmann-MIT Bridge Program; the Schwartz/Reisman Collaborative Science Program; the Andre Deloro Prize for Scientific Research; the Ben B. and Joyce E. Eisenberg Foundation; and Miel de Botton.






