EMBL Hamburg and CSSB scientists have uncovered the molecular details of how we absorb vitamin B1, paving the way to preventing dangerous hidden B1 deficiencies in patients
Summary
- Vitamin B1 deficiencies can have devastating health consequences.
- EMBL Hamburg's Löw Group provided detailed molecular insights into how specialised transporter proteins enable vitamin B1 to cross membranes in our body to reach vital tissues and organs.
- They identified several common drugs that disrupt vitamin B1 transport, potentially causing deficiency of this nutrient in the brain despite normal blood levels. The finding could help prevent this side effect in the future.
- The study offers also new insights into rare diseases of the nervous system caused by impaired B1.
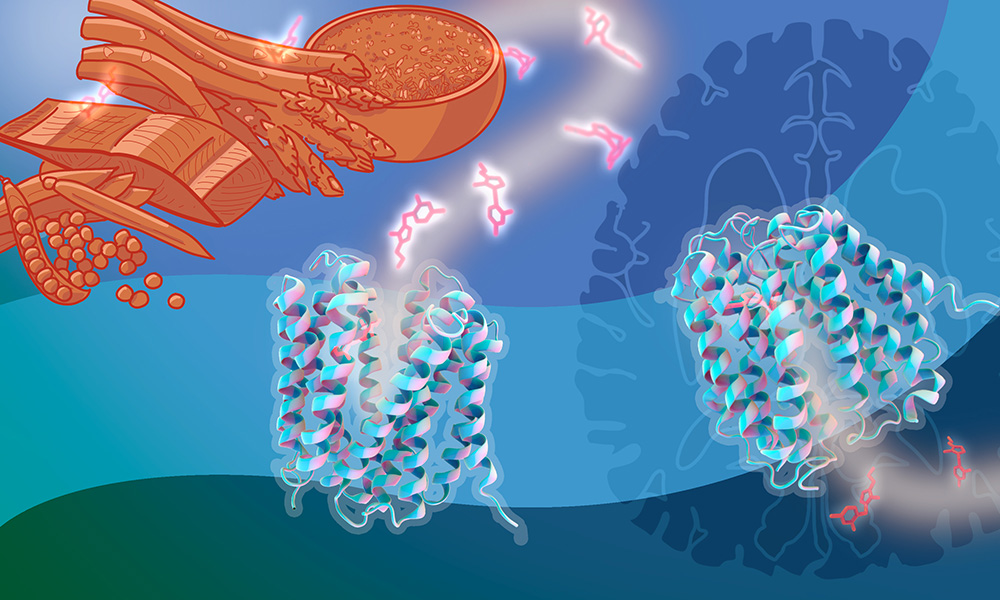
Vitamin B1, also known as thiamine, is essential for the survival of our cells. The human body can't produce it, but we can maintain healthy levels of this vitamin by eating foods like salmon, legumes, and brown rice. Doing this is crucial, because B1 deficiency can cause serious dysfunctions of the cardiovascular and central nervous systems, disability, and even death.
However, sometimes, B1 deficiency may develop in the brain and other vital organs as a side effect of some drugs. This can happen despite normal B1 levels in the blood, which often makes such deficiencies go undetected before it's too late.
To understand what's behind such hidden deficiencies, the Löw Group at EMBL Hamburg and CSSB and collaborators at VIB-VUB Center for Structural Biology used structural biology and biophysical techniques to investigate how vitamin B1 travels in our body to reach different tissues, and what factors can hinder its progress.
Vitamin B1's hurdle run
On its journey from the gut to the body's cells, vitamin B1 must pass through several membranes, which act as barriers - starting with the gut wall, then blood vessels, organs, and finally, the membranes of individual cells. The most stringent of these is the blood-brain barrier, which seals the brain off from toxins that might enter from the bloodstream. However, the barrier also makes it difficult for essential nutrients, including vitamins, to cross.
To allow vitamins and other nutrients to reach cells throughout the body, these membranes are equipped with specialised transporter molecules that let them pass. In vitamin B1's case, this job is done mostly by two transporters: SLC19A2 and SLC19A3. While we know these transporters are important for human health, it has been unclear how exactly they work on the molecular level.
To uncover this, the Löw Group investigated SLC19A3, the transporter essential for getting B1 across the gut wall and the blood-brain barrier - two crucial steps in the vitamin's journey.
To observe the transporter in action, they created a 'molecular movie' by putting together a series of snapshots obtained with cryo-electron microscopy (cryo-EM).
"With this, we could capture the dynamics of the transport process and visualise molecular details of how the transporter recognises and pushes the B1 molecule across the cell membrane," said Christian Löw, Group Leader and corresponding author of the study.
Insights into rare diseases
The molecular snapshots enabled the scientists to determine which parts of the SLC19A3 transporter are the most critical for it to work correctly. If these parts malfunction, the transporter won't work.
This explains why mutations in these critical parts impair B1 transport to the brain and lead to severe neurological symptoms. These rare conditions , which start manifesting symptoms in infancy, are treated with high doses of B1 and other compounds. Despite this, one in 20 patients die and nearly one-third still suffer from symptoms.
To investigate this, the scientists created a version of the SLC19A3 transporter carrying a mutation that causes a severe brain disease called BTBGD . This let them observe exactly how the mutation affects the transporter's molecular structure and makes it unreceptive to B1. Understanding this disease-causing mechanism might help to design more effective treatments for BTBGD in the future.
Drugs that can cause hidden B1 deficiencies
Severe B1 deficiency symptoms can be caused not only by rare mutations, but also by some medications. Several commonly prescribed drugs, including some antidepressants, antibiotics, and oncological medications, impair SLC19A3. This can potentially lead to dangerous B1 deficiencies throughout the body or in specific organs or tissues.
Brain-specific deficiencies are especially dangerous because they can occur even when our blood levels of B1 are normal, making them undetectable by standard blood tests. This hidden deficiency can quietly lead to serious, potentially fatal brain dysfunction.
"While medicine already knows a few drugs that have the potential to cause hidden B1 deficiencies, there may be many more that we're unaware of," said Florian Gabriel, PhD student at EMBL Hamburg and the first author of the study. "Identifying them isn't straightforward, so our research aimed to make it easier. We've uncovered the molecular basis of how drug molecules block the SLC19A3 transporter and we are currently using that knowledge to screen all FDA - and EMA -approved drugs for similar effects."
The Löw Group also identified the structural features that make a drug likely to impair B1 transport. To do this, they used cryo-EM and biophysical techniques to analyse how known blockers interact with SLC19A3.
Using this knowledge, they have identified seven new drugs that block the B1 transporter in vitro and are likely to do it in the human body as well. These include several antidepressants, the antiparasitic hydroxychloroquine, and three cancer drugs.
While these findings still need to be confirmed in humans, they are a first step to protect patients from potentially dangerous drug-induced B1 deficiencies in the future.
"These results will not only help to better monitor the health of patients taking those drugs, but might also help to design new drugs in the future that won't have this side effect," said Löw. "We believe our work could also create a basis for studying how medicines interact with similar transporters in the human body. In the long term, it might also guide the design of future drugs that could use those transporters to reach target organs more efficiently."
For solving the structures of the SLC19A3 transporter, the Löw Group used the state-of-the-art cryo-EM facilities at the CSSB in Hamburg and the EMBL Imaging Centre in Heidelberg. Critical biophysical experiments were performed at EMBL Hamburg's Sample Preparation and Characterisation Facility .






