As nations around the globe consider ways to stave off climate change, the United States in 2022 passed legislation to invest billions in new green energy technologies: solar and wind power, new transmission grids, electric vehicles and advanced battery storage systems.
It sounds promising, but we've got a long way to go. Despite producing more and more renewable energy each year, the United States still gets only 9.2% of its electricity from wind and only 2.8% from solar. About 61% of our electricity comes from fossil fuels, with roughly two-thirds of that coming from natural gas and one-third from coal, according to 2021 estimates by the U.S. Energy Information Administration.
The road to a clean, green energy future is long with many twists and turns, U-turns and obstacles, but it's one that Washington University researchers are traveling in hopes of building better and more sustainable batteries. In Vijay Ramani's lab, for example, scientists are working on large-scale batteries, the ones that must be built into the power grid so that we can actually make use of our abundant wind and solar resources. And in Peng Bai's lab, they are looking at batteries that take a sip from that grid and then set us free: the batteries in our electric vehicles and mobile devices.
While green energy may be the only viable solution to a looming climate catastrophe, using current technologies presents us with tough decisions and unpleasant trade-offs.
"I would love to have better energy technologies deployed right now. But as a chemical engineer who understands … scale … it's going to take some time before we
have that."
Vijay Ramani
For starters, many raw materials critical to green energy technologies are already in short supply and are extracted through unsustainable and decidedly environmentally unfriendly mining processes. Surging global demand for key battery components -- such as lithium, graphite, nickel and cobalt - has sparked a wave of new mining proposals aimed at extracting critical minerals from remote, environmentally fragile corners of the globe.
Without rapid breakthroughs, the ecological and human health consequences of green energy development could rival those left behind by centuries of reliance on coal, oil and gas.
"There's no silver bullet that's going to provide a quick fix," says Ramani, a professor of energy, environmental and chemical engineering in the McKelvey School of Engineering. "I work in renewable energy, and I've worked with batteries all my life. I would love to have better energy technologies deployed right now. But as a chemical engineer who understands the concept of scale, I can guarantee you that it's going to take some time before we have that.
"It's a complex game. The economics have to work out."
Power when it's needed most
Ramani, who also serves as WashU's vice provost for graduate education and international affairs, has been exploring alternative energy since 2001 when he became fascinated with hydrogen fuel cells as a doctoral student in chemical engineering at the University of Connecticut.
He continued his green energy research as a professor at the Illinois Institute of Technology until 2016, when he joined WashU as the Roma B. & Raymond H. Wittcoff Distinguished University Professor.
Ramani is one of many researchers at McKelvey pursuing new technologies to reduce harmful effects of fossil fuel emissions and speed the transition to cleaner, more environmentally friendly forms of energy generation, storage and transmission.
Specifically, he and his McKelvey colleague Bai, an assistant professor also in the energy, environmental and chemical engineering department, are focused on an area of research that is pivotal to the success of the green energy revolution: the critical need for more –
cost-effective and sustainable battery technologies. Each directs a team of about 10 researchers, mostly doctoral students and postdoctoral research assistants, who are training for careers in the green energy sector.
Ramani's lab is exploring development of massive, grid-scale batteries that will be essential for the longer-term storage of electricity generated from wind and solar. Unlike the baseline electricity from fossil fuel and nuclear power plants, which can be generated continuously, power from wind and solar is intermittent, meaning it must be used immediately to meet consumer demand or somehow stored for later use. Dark nights and calm days can make it difficult or impossible to generate, transmit and distribute power from renewable sources reliably, so mega-capacity, grid-level storage will become more and more necessary as we move away from fossil fuels.
Ramani and his research team, including some of his current and former students, now hold patents on several innovations that pave the way for the commercial rollout of new, higher-performance versions of these large batteries. Their battery solution, which uses titanium and cerium to replace the costly vanadium used in most near-market redox flow technologies, has the potential to meet federal cost-efficiency standards for grid-scale energy storage systems. His team recently received a $2 million federal grant to explore how the invention could be derisked as a prelude to scale-up for commercial applications.
"Solar energy generation has become cheap, as has wind energy. But you can't always rely on either source to generate power when you might need it most," Ramani says. "You have to figure out some way to store massive amounts of energy for days at a time, because there's no way you can run an entire power grid on intermittent energy sources. It's impossible."
Grid-scale battery solutions like Ramani's would give us a way to fill in the gaps.
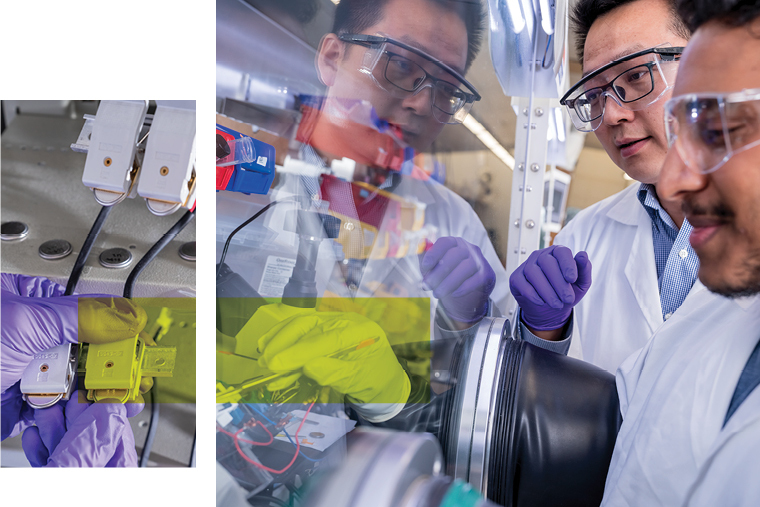
Smaller batteries need to step up, too
On the other end of the rechargeable battery spectrum, Bai and his team are focused on the slim, compact batteries that power our mobile phones and laptops; the chunky, block batteries behind our cordless power tools; and the still larger, multi-cell battery packs featured in our emission-free electric vehicles.
Bai earned a bachelor's degree in automotive engineering in 2007 and a doctorate in mechanical engineering in 2012, both from Tsinghua University in China. He also studied chemical engineering for several years as a research scientist at Massachusetts Institute of Technology.
Since joining WashU in 2017, his research has explored cleaner, more efficient and safer alternatives to lithium-ion batteries, now one of the most common technologies in the mobile battery marketplace.
Lithium-ion batteries, first commercialized in the 1990s, have a reputation for dependable service over many use cycles. But these advantages come with a battery chemistry that relies on an array of minerals that can be difficult to extract and resource-intensive to process.
Among the most problematic components of these standard types of batteries: the lithium itself, which is harvested from brines pumped from groundwater under the Andes Mountains of South America, and graphite, which is mined almost exclusively in China. Refining graphite requires extreme heat, often supplied by burning energy from coal-fired power plants, and treatment with hydrofluoric acid, a process that can be dangerous to surrounding ecosystems.
"Although lithium-ion batteries hold the promise to decarbonize transportation, the mining and processing of battery-grade graphite at the scale of close to a million metric tons each year have strained our ecosystems through pollution and greenhouse gas emissions," Bai says. "The situation will only get worse with the rapid expansion of battery production worldwide - until we take a simple action: removing graphite from batteries."
"We are investigating how lithium batteries can be improved or how alternatives to them can be developed, so we can produce energy-dense batteries that are both safer for the user and more sustainable for the planet."
Peng Bai
Bai's lab has made recent discoveries that accelerate doing that and more - developing a lithium-ion battery that works without graphite and a sodium-ion battery that works without lithium. Bai and others have found potential substitutes scattered across the periodic chart.
"You can make good batteries by incorporating lithium, graphite, cobalt, nickel and other minerals that are not so abundant, but it will have an environmental and social price," Bai says. "We are investigating how lithium batteries can be improved or how alternatives to them can be developed, so we can produce energy-dense batteries that are both safer for the user and more sustainable for the planet."
U.S. legislation is driving changes
In the U.S., decades of improved environmental regulations and green activism have forced many of our dirtiest mining and refining operations to move overseas, effectively outsourcing the ecological and social costs of battery production.
In 2022, President Joe Biden signed Democrats' landmark climate change and health-care bill into law. Among many other things, the Inflation Reduction Act (IRA) aims to reverse that outsourcing trend by offering generous consumer rebates for the purchase of electric cars, batteries and other green technologies with components substantially mined or manufactured in the U.S.
Michael Wysession, professor of earth and planetary sciences in Arts & Sciences and an expert on energy use, is so confident that the IRA will motivate big changes that he dubs 2023 "The year of the battery."
"President Biden's centerpiece legislation is a game-changer in many areas of renewable energy," Wysession says. "One of the major challenges to a fully renewable-energy future of wind and solar power is energy storage. Just a few years ago, people were looking toward large earth-works projects, such as pumped hydro or compressed air in underground caverns. Those discussions have been largely shelved because of the incredibly rapid advances and dropping prices of battery technologies."
For example, from 2010 to 2022, the cost for enough electric vehicle lithium-ion batteries to store a kilowatt-hour (kWh) of energy (which is the equivalent of 10 100-watt lightbulbs burning for an hour) dropped from $1,300 to just $150, in 2022 dollars, Wysession notes.
"The IRA increases the investment tax credit to 30% for solar-plus-storage and, for the first time, standalone storage facilities," Wysession says. An extra 10% credit is added for using equipment manufactured in the U.S., and another 10% is added for projects located at decommissioned fossil fuel facilities in front-line communities.
"This will counteract the 2021-22 supply chain bottlenecks and help spur U.S. development," Wysession continues. "Since the passage of the IRA, several major battery manufacturers, such as Panasonic and LG, have already announced plans to build factories in the U.S."
Ramani is less sanguine about the immediate changes to expect, but he still sees great opportunity in battery and energy storage research and development in coming years.
Great grids
On the bright side, both wind and solar power can be cheaper to produce than power from natural gas, so utilities have an economic incentive to consider making the switch. Many utilities are eager to use renewables in the grid in whatever increments they become available, especially for replacing the energy that comes from peaking power plants that generally run only when there is a high demand for electricity.
"You can't run an electric grid with this sort of uncertainty, though," Ramani says. "You can use intermittent sources on a percentage basis as long as it's less than 10%, 15%, 20%. The existing grid has enough flexibility to take it when it comes and leave it when it doesn't. But if you want 90% solar and wind, as some folks are proposing, that's not going to happen unless you have a storage technology."
And the storage capacity needed for a 100% renewable energy grid would be truly enormous. "I'm not talking kilowatts or megawatts or 1,000 megawatts, which is a gigawatt. I'm talking hundreds of gigawatts and thousands of gigawatt hours," Ramani says. "That's the scale of storage we'll need for a renewable-based electric grid."
Right now, the world's top 10 largest power station batteries are all built using lithium-ion chemistries, which have relatively short charge-discharge cycles. Most provide limited back-up power for several hours or less to meet small surges in demand or respond to emergencies when a storm or wildfire takes down parts of the grid.
"That's not near long enough for a renewable energy grid," Ramani says. "For renewables, you need a system that can store at least 8 to 10 hours, enough to get solar power through the night, especially if everyone's home charging their electric vehicles."
Redox flow batteries, such as the one recently patented by Ramani and his group, could help meet the demand for large-scale storage. Their design creates energy-dense liquid electrolytes that can be pumped into nearby storage tanks. Energy-holding capacities can be scaled simply by increasing the size or number of storage tanks. Prototype systems prepared by companies working in this space, which are now about the size of a shipping container, could be expanded to store energy in tank farms as large as sports arenas.
Ramani's flow battery uses a combination of titanium and cerium metals, which are more abundant and cheaper than the vanadium used in other, more established, flow battery chemistries. It may be a few years before their titanium-cerium flow battery is ready for large-scale commercial applications, but Ramani and his colleagues are hoping to have a pilot version scaled up and ready for demonstration within a year. His group, along with Ben Kumfer, a research assistant professor and colleague, are working with the St. Louis-based electrical utility Ameren to model the use of such batteries in conjunction with gas-fired power plants, an exciting local project that is funded by a two-year, $500,000 federal grant.

Next-gen batteries for cars
Bai's research aims to better understand the chemical reactions, known as thermal runaways, that can cause energy-dense lithium batteries to heat up uncontrollably. In rare cases, these reactions have triggered dangerous battery explosions and fires in vape pens, mobile phones, laptops and electric vehicles.
By listening to the electrochemical and physical forces at the heart of battery functions, his team has documented subtle mechanisms that determine how these reactions play out at the level of individual particles, ions and electrons.
Working with Bingyuan Ma, one of his doctoral students, Bai has developed a transparent capillary cell that allows spider web- thin working models of simple, single-cell batteries to be observed under a microscope in real time. The research links some little understood battery failures to chemical reactions that can be prevented with subtle changes in battery design and materials.
As detailed in a paper that Bai and Ma published in 2021, their lab has used these insights to develop a stable sodium-ion battery prototype with potential to end battery industry reliance on both lithium and graphite. Since traditional lithium-ion batteries use 10 to 30 times as much graphite as lithium, graphite-free sodium batteries have the potential for much higher energy densities, allowing even more energy to be packed into a battery of similar size and weight. Since sodium, the same element in table salt, is both abundant and cheap, the raw material costs for sodium batteries would be less than 1% of those for lithium batteries.
Ma, who completed an internship in battery development with Tesla, has an offer to re-join the electric vehicle (EV) manufacturer when he completes his doctoral degree. Another of Bai's students, Shubham Agrawal, who graduated from WashU in 2022 with a doctorate in chemical engineering, now works as a battery modeling engineer with the EV division of Ford Motors.
Agrawal is often surprised at how little most consumers seem to know about the basic workings of EVs. Many don't realize that EV batteries often comprise as many as a thousand small, AA-size batteries cobbled together into computer-monitored, multi-cell packs. Nor do many understand that EV batteries are mounted along the car's chassis at wheel level, leaving the area under the front hood once occupied by an internal combustion engine to be used as extra trunk space.
"Our car and battery companies are bringing these innovations directly to their production lines, so that's where our students' precise understanding of electro- chemical science can play a significant role."
Peng Bai
Agrawal, who evaluates battery technologies as part of his job at Ford, says the lithium-ion batteries he studied in Bai's lab will remain the major energy source for commercial applications for several years. However, Ford and other manufacturers, he notes, keep an eye out for upcoming options to provide even safer and cheaper batteries than the now most popular nickel-manganese-cobalt-based batteries. He expects EV performance to improve dramatically as new advances come online.
"In the next five years, you will definitely see EVs going 600 or 700 miles on a single charge," Agrawal says. "Charging times will be reduced to 15 to 20 minutes, and battery life definitely will increase as promising new chemistries come online. Even the charging stations are becoming more available, so I think the customers will be very happy with the progress we're making."
Bai says his doctoral students leave the university well qualified to become professors and pursue careers in academia, but he also encourages them to consider jobs in industry. "Our car and battery companies are bringing these innovations directly to their production lines, so that's where our students' precise understanding of electrochemical science can play a significant role," Bai says. "That's where the fundamental science behind next-generation batteries will really be implemented."
Making the case for transition
Governments around the world have a huge role to play in expanding and encouraging a transition to renewable energy. They could choose to expedite the transition, too, by taxing fossil fuels, subsidizing renewables and allowing public utilities to raise rates to cover switchover costs. But Ramani has concerns about how quickly these changes can occur, and about how realistic it is to expect countries like India and China, with a high growth trajectory, to participate at this point.
"If you want to replace all fossil-based assets with solar and wind and storage, that's an awful lot of investment, and that's my worry," Ramani says.
Ramani expects the renewable energy transition to happen gradually over decades. How fast it happens will depend almost entirely on how average consumers view the comparative cost of renewable options.
"I think it's important that we start moving in the right direction as fast as we can, as rationally as we can. But I think it's equally important to be as honest as we can with people as to the pros and cons of our current green energy technologies," Ramani says.
"We need to look at all these alternative technologies and think productively about how we can bring them to fruition," he says. "We need to paint the whole picture, let the chips fall where they may, and the most cost-competitive technologies will win. In the end, it's the economics that will dictate where we end up."






