Researchers at the University of Tübingen have developed a new method to better study atheroscle-rosis in mice. The non-invasive imaging method helps to better understand and treat narrowing of blood vessels, a cause of heart attacks and strokes. The new approach may also significantly reduce the number of animals used in experiments compared to previous methods. The results were published in the journal Circulation Research.
Atherosclerosis, also known as "calcification" of the arteries, can lead to heart attack or stroke and is the leading cause of death worldwide. Several factors, such as high cholesterol, can contribute to the development of pathological changes in arteries called plaques. These lesions often severely narrow blood vessels or even lead to the formation of blood clots, so that the heart or brain are no longer adequately supplied and are damaged as a result of oxygen deficiency.
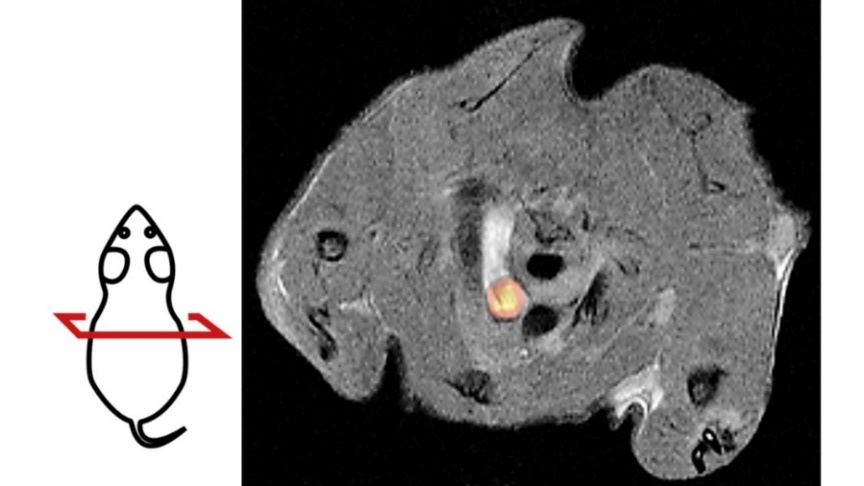
The new method for studying atherosclerosis is based on an artificial positron emission tomography (PET) reporter enzyme. This is specifically produced in mouse vascular muscle cells by a genetic trick. It causes the accumulation of a radioactive substance, the PET tracer, in these cells. The radioactive substance, which is harmless to the animal, is detected by PET and visualized on a screen. PET is a clinically established method that is used, for example, to examine tumor patients. As a non-invasive technique, PET imaging puts less strain on the organism than many other examination procedures.
By combining PET with magnetic resonance imaging (MRI), the research team is now able to track the position and number of vascular muscle cells in the body. "This method allows us to observe in living animals how the labeled cells are involved in the development of atherosclerosis," explains first author and study leader Dr. Susanne Feil from the Interfaculty Institute of Biochemistry (IFIB) at the University of Tübingen. For example, it is possible to see where vascular muscle cells accumulate in blood vessels and contribute to the development of plaques. By visualizing such cell accumulations, conclusions can be drawn as to whether changes are harmless or could have life-threatening effects, for example because they could lead to vascular occlusions and infarcts (see figure).
"Furthermore, the new PET method requires significantly fewer test animals in comparison with previous techniques," the research team says. The labeled cells - and thus also the development of ath-erosclerosis - can be followed non-invasively over many weeks in the same animal. According to the researchers, such long-term studies allow significantly more data per animal to be obtained, which are also of better quality, since there are no interindividual variations in the measured values.
"This is in line with the 3Rs principle," says Feil. "The aim is to avoid animal experiments as far as possible (replacement) and to keep the number of animals (reduction) and their strain (refinement) in experiments as low as possible. To analyze the behavior of cells (e.g., vascular muscle cells) in mice, methods involving a relatively high burden or use of large numbers of test animals have been employed in the past."
The new PET method was developed in collaboration between the Interfaculty Institute of Biochemistry and the Werner Siemens Imaging Center of the Tübingen University Hospitals. The project was funded by the EU, the German Research Foundation (DFG) and the Dr. K. H. Eberle Stiftung.
Publication:
Feil S, Stowbur D, Schörg B, Ehrlichmann W, Reischl G, Kneilling M, Pichler B, Feil R. 2023. „Non-invasive detection of smooth muscle cell-derived hot spots to study atherosclerosis by PET/MRI in mice". Circulation Research, doi.org/10.1161/CIRCRESAHA.122.322296
and https://www.ahajournals.org/doi/abs/10.1161/CIRCRESAHA.122.322296