Catalysts usually consist of valuable noble metals, which are rare and not available in unlimited quantities. Therefore, it is important to optimize the catalyst for the respective reaction. The group of Jeroen van Bokhoven was now able to study a catalyst in atomic resolution and real time, and thereby gaining important new insights.
Heterogeneous catalysis is a key process in the chemical industry, as more than 80% of all manufactured chemicals have a catalytic process involved during their production. Thus, a key towards the transition to a sustainable and environmentally friendly society is the optimization of such industrial processes, also in terms of energy efficiency. The precious metal platinum - of which the world's resources are scarce - is an important and frequent component of catalysts. In order to effectively improve catalysts, it is a prerequisite that the mechanism of the process is known - otherwise "the improvement of catalysts simply continues to be subject to trial and error", as Arik Beck, first author of the study which was recently published in Nature Communications, describes it. This is exactly what Jeroen van Bokhoven's group has now achieved - in collaboration with Xing Huang and Marc-Georg Willinger from ScopeM, the Scientific Center for Optical and Electron Microscopy at ETH Zurich.
Using high-resolution electron microscopy, they were able to monitor the catalytic process live, i.e. in-situ, and gain important new insights into the course of the reaction. Video recordings in atomic resolution make it possible to observe and understand the various process steps in real time. And even individual platinum atoms are visible.
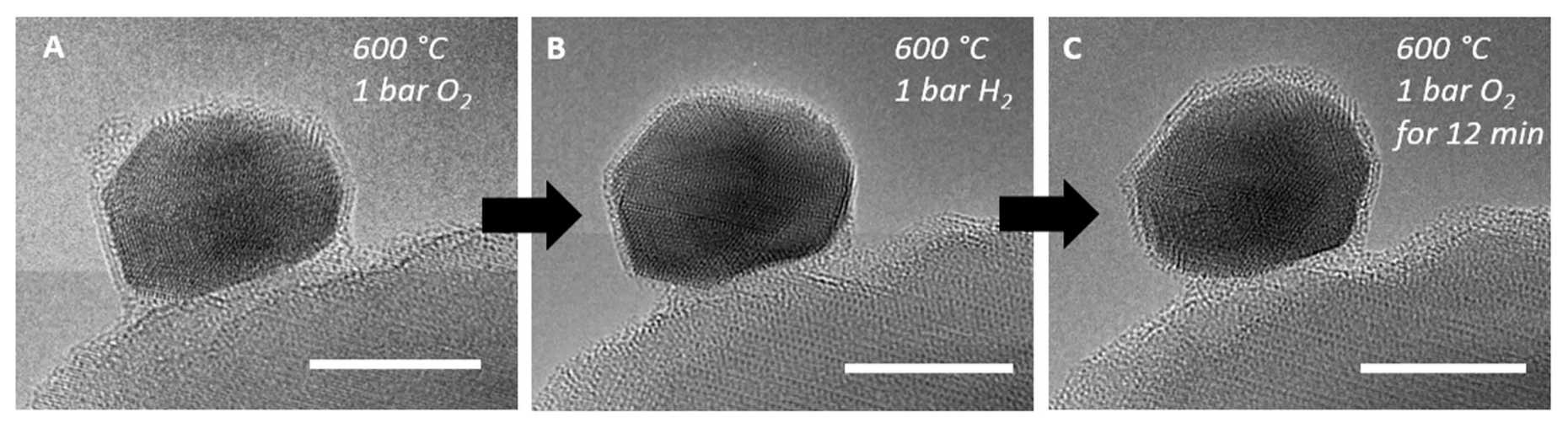
As shown above, a platinum nanoparticle (dark area in the center of the images) is sitting on a titanium dioxide carrier. As the gas atmosphere changes from hydrogen to oxygen, the surface structure of the platinum particle changes. The white bar corresponds to a length of 5 nm, for comparison: the diameter of a hair is about 50 000 nm. These video recordings were made in collaboration with ScopeM, the Scientific Center for Optical and Electron Microscopy at ETH Zurich.

For catalytic processes, platinum is usually distributed as very small nanoparticles (1 to 10 nm) on a metal oxide material as a carrier, the so-called support. In the early days of catalysis, it was assumed that these support materials would act as inert carrier materials. Already 40 years ago, it could be shown, that the support materials are anything but inert, and the phenomenon of a strong interaction with the support material was observed, which was named Strong Metal-Support Interaction (SMSI). This effect plays an important role in catalysis, as it has a substantial influence on the selectivity and efficiency of the process.
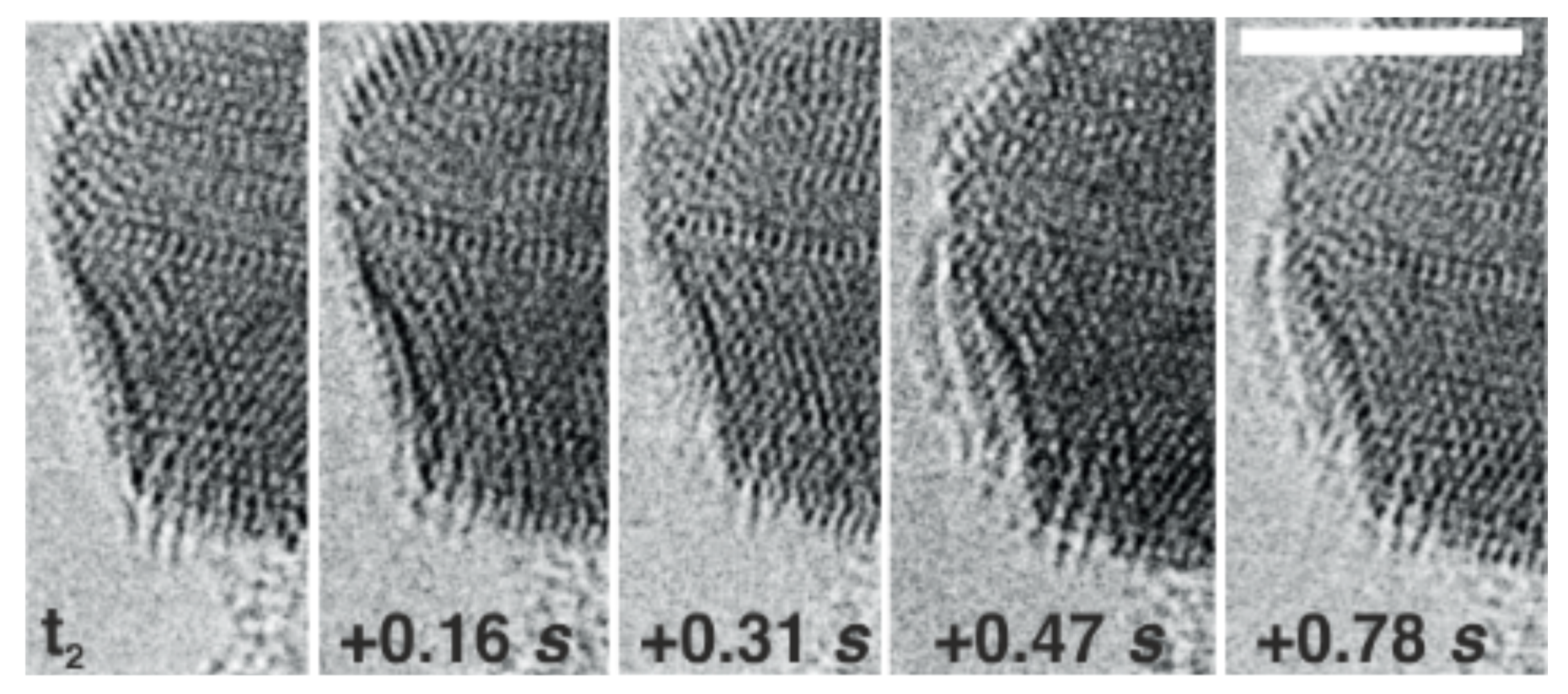
As the images above show, a layer of titanium oxide suddenly forms - in less than a second - on the platinum particle. Here you can see how the atomic lattice is first restructured on the side of a particle and then, between 0.31 sec and 0.47 sec, a bright structure suddenly appears on the side. In electron microscopy, bright structures indicate that the atoms have a low mass. Thus, one can distinguish between platinum (heavy and therefore dark) and titanium oxide (light and therefore bright). The grid-like structure that can be seen in the images is the atomic lattice structure. Each point corresponds to an atomic position in crystal lattice.
Metal oxide support materials which are easy to reduce, such as e.g. titanium oxide, can be modified by reductive treatment with hydrogen, making them more efficient for certain catalytic reactions. These modification processes involve the formation of thin layers of the support material on the surface of the platinum nanoparticles, a so-called encapsulation. However, this effect has never been fully understood, as samples of the catalysts taken out from the reactor immediately change, even before further investigation.
Due to ScopeM's state-of-the-art transmission electron microscopy, the process of overlay formation can now be observed and analyzed in-situ - in atomic resolution and in real time. For the first time, it was possible to observe how the platinum atoms of titanium oxide are encapsulated at high temperatures and how this surface structure changes when the gas, which surrounds the system, is exchanged.
These videos of the process steps provide unprecedented insight into this process and showed that in a competing process also a platinum-titanium alloy is formed. Surprisingly, it turned out that the overlayer formed is stable even in the presence of oxygen and at high temperatures - something that was previously unknown. This gives rise to the hope that this reaction can now also be used for catalytic oxidations, thus opening up new application potential in catalysis and materials science.
Jeroen Bokhoven and his team are pleased that this combination of the latest in-situ techniques has elucidated an important industrial reaction mechanism, providing a holistic and complementary view of catalytic reactions.
Reference
Beck A, Huang X, Artiglia L, Zabilskiy M, Wang X, P Rzepka P, Palagin D, Willinger M-G, Bokhoven JA. The dynamics of overlayer formation on catalyst nanoparticles and strong metal-support interaction. Nature Communications 2020 11:3220. DOI: 10.1038/s41467-020-17070-2






