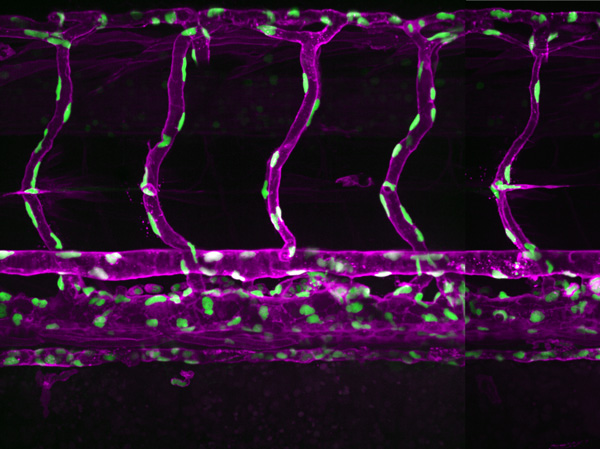
Figure 1: A confocal light micrograph showing blood vessels in a three-year-old zebrafish (magenta: endothelial cells of blood vessels; green: endothelial cell nuclei). RIKEN researchers have shown that water flow plays a role in the development of new blood vessels in zebrafish. © 2025 RIKEN Center for Biosystems Dynamics Research
Water flow plays a surprising role in the formation of new blood vessels as zebrafish develop, RIKEN researchers have discovered1. This finding advances our understanding of how blood vessels sprout new branches through cell migration.
Blood vessels supply the cells in our bodies with the oxygen and nutrients they need to function and grow. Especially during development, blood vessels sprout new branches to supply blood to new areas. Tumors hijack this process to syphon off resources to support their proliferation.
New branches form through special cells at the leading edges of blood vessels-endothelial tip cells-migrating to new locations, where they form vascular sprouts. Efficient cell migration is driven by a process known as actin polymerization in which the building blocks of the polymer actin join together to form filaments.
Now, a team led by Li-Kun Phng of the RIKEN Center for Biosystems Dynamics Research has discovered another mechanism is also at work-they showed that water flow also assists endothelial tip cells to migrate in zebrafish.
"The fact that water flow and the build-up of hydrostatic pressure help endothelial tip cells to migrate is an exciting find," says Phng. "Most vascular biologists thought that actin polymerization is the only driving force for cell migration, but we've now shown that another process is also at play."
The team knew there had to be another process, because endothelial tip cells still migrated when they turned off actin polymerization in zebrafish in an earlier study.
In the present study, they showed that water flow is the second mechanism by generating zebrafish lacking genes that code aquaporins-channels that facilitate the movement of water in to and out of cells. When they did this, they observed defective migration of endothelial cells in the zebrafish.
"We found that when there are no aquaporins, meaning there's no water flow into endothelial cells, there is defective endothelial tip cell migration," says Phng. "And when we inhibited actin polymerization in aquaporin mutants, there was greater inhibition of endothelial tip cell migration. Combining these two results therefore shows that there are two mechanisms for endothelial tip cell migration during development in zebrafish."
"How endothelial cells migrate is very important because new blood vessels won't form if they fail to migrate," adds Phng. "Or if they migrate in a perturbed manner, mispatterned blood vessels can form, which can lead to problems with blood flow and poor perfusion of tissues."
The existence of two mechanisms means there is a backup if something goes wrong. "If one mechanism fails, the other mechanism will still be running to ensure that blood vessels form," notes Phng.

Li-Kun Phng (fourth from left) and her team have shown that that hydrostatic pressure is one of two mechanisms that control endothelial cell migration during angiogenic sprouting in zebrafish. © 2025 RIKEN






