A new study has challenged conventional wisdom on oxygen reduction reaction (ORR) catalysts by uncovering a novel reaction pathway in weak-binding metal-nitrogen-carbon (M-N-C) single-atom catalysts (SACs). These findings could revolutionize the design of next-generation electrocatalysts for clean energy applications.
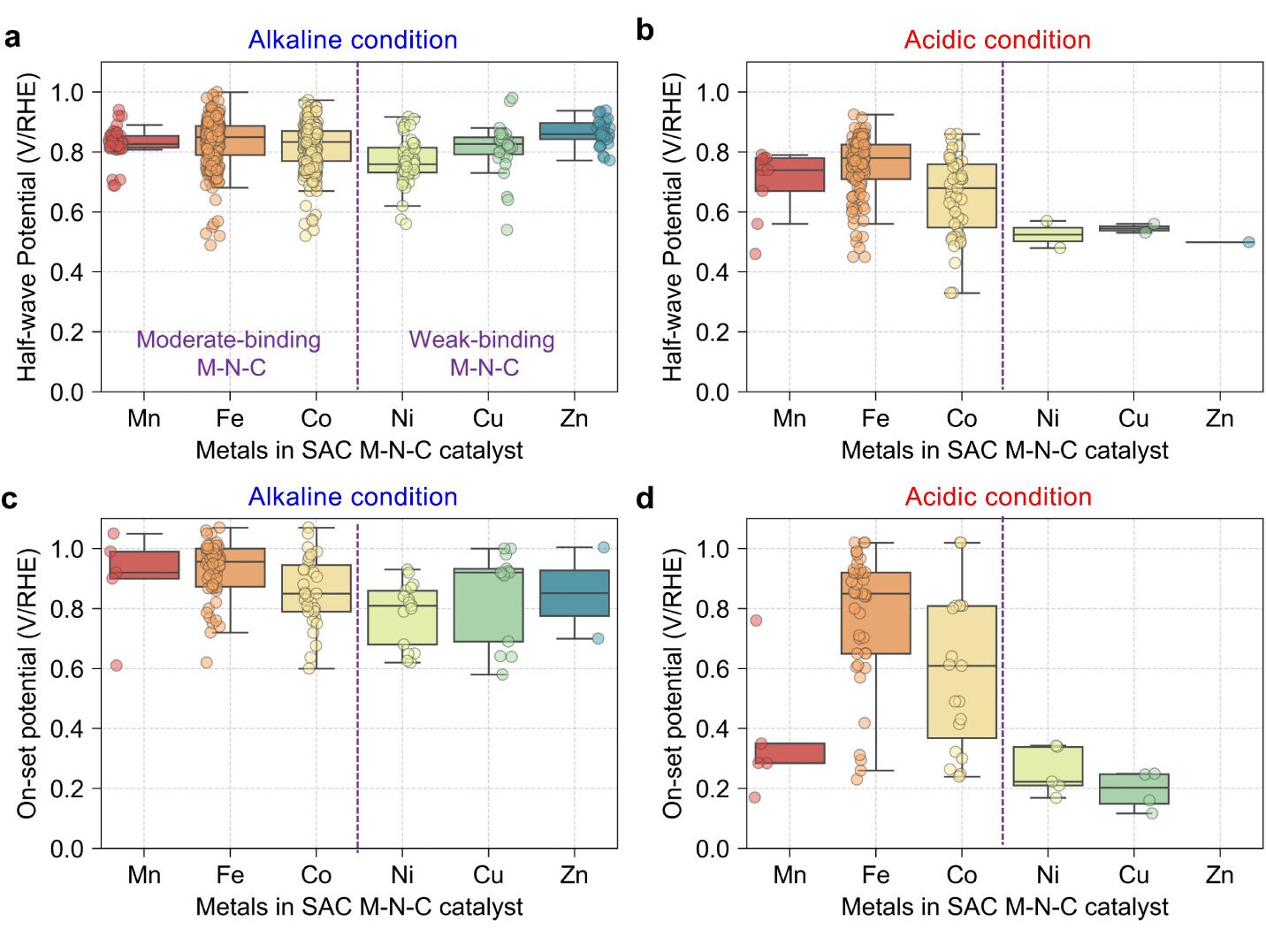
Traditionally, optimal ORR catalysts were believed to follow the Sabatier principle, where moderate binding strength at metal active sites enhances catalytic performance. However, weak-binding SACs, such as Ni-N-C and Cu-N-C, exhibit unexpectedly high ORR activity, contradicting this principle.
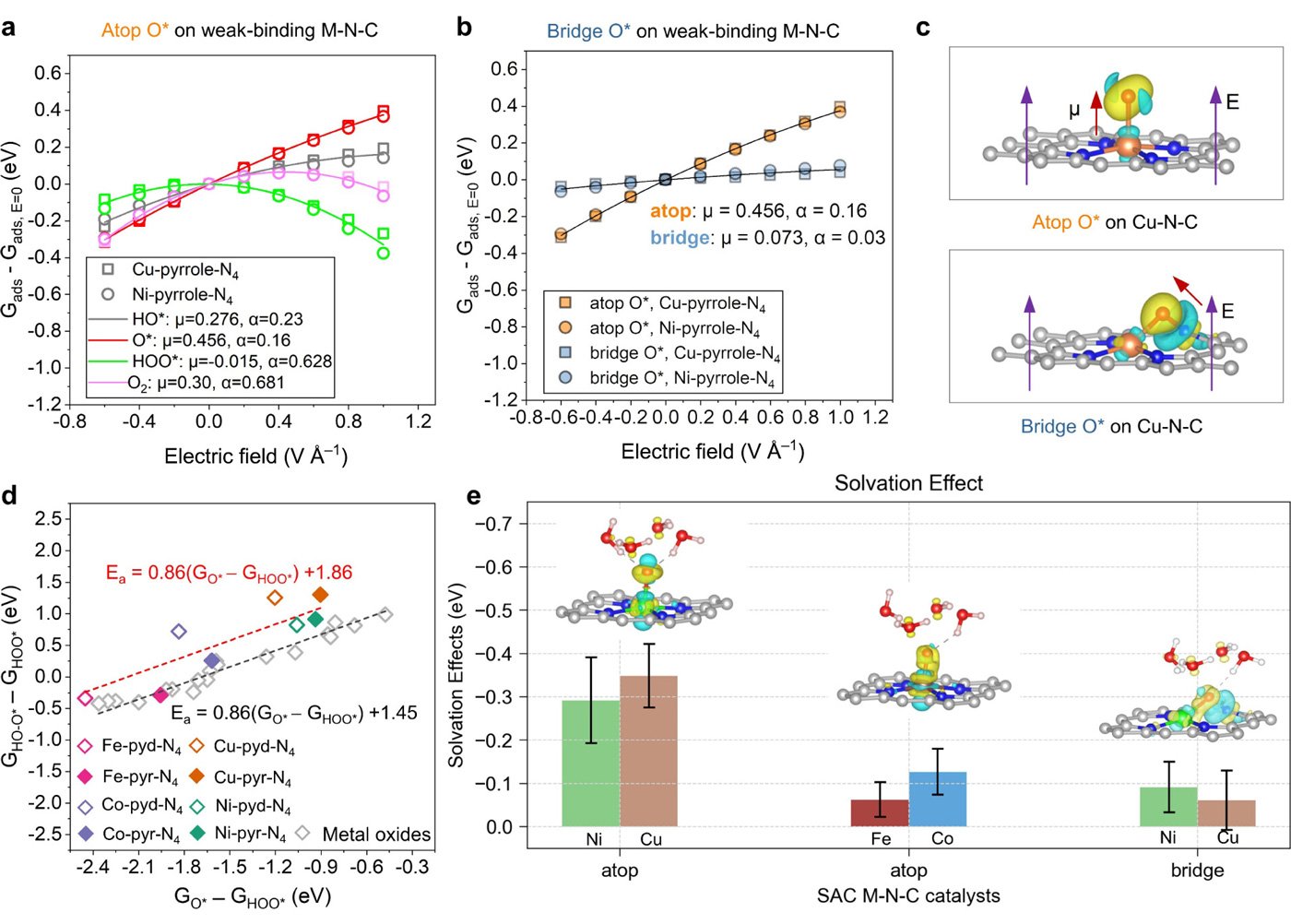
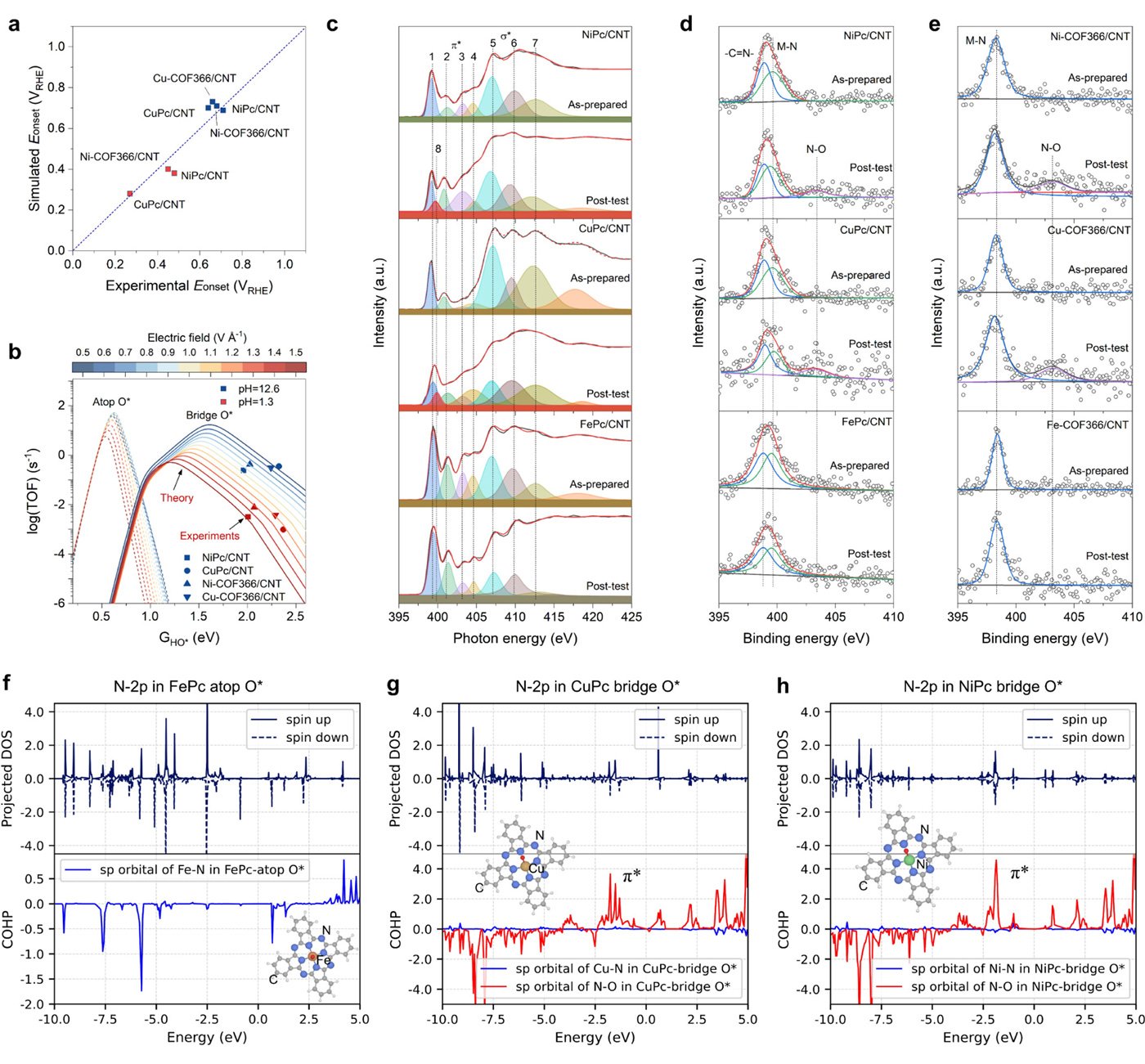
By integrating a pH-dependent microkinetic model with advanced synchrotron spectroscopy, the research team discovered that atomic oxygen (O*) adsorption at the metal-nitrogen bridge site--rather than the conventional metal atop site--is a crucial step in the ORR mechanism for weak-binding catalysts. This adsorption alters key reaction barriers and scaling relations, offering new insights into their exceptional catalytic behavior.
"These results not only redefine our understanding of weak-binding M-N-C catalysts but also provide a new strategic direction for catalyst design," said Di Zhang, an assistant professor at Tohoku University's Advanced Institute of Materials Research (WPI-AIMR). "By leveraging our findings, we can optimize weak-binding M-N-C catalyst structures for improved performance across different pH environments."
Looking ahead, the research team plans to integrate machine learning force fields with studies on new reaction pathway exploration to accelerate the prediction and design of high-performance electrocatalysts.
The structures in this research have been made available through the Digital Catalysis Platform (DigCat), the largest experimental catalysis database to date, developed by the Hao Li Lab.
Details of its findings were published in the Journal of the American Chemical Society. The article processing charge (APC) was supported by the Tohoku University Support Program.
- Publication Details:
Title: Why Do Weak-Binding M-N-C Single-Atom Catalysts Possess Anomalously High Oxygen Reduction Activity?
Authors: Di Zhang, Fangxin She, Jiaxiang Chen, Li Wei, Hao Li
Journal: Journal of the American Chemical Society
DOI: 10.1021/jacs.4c16733






