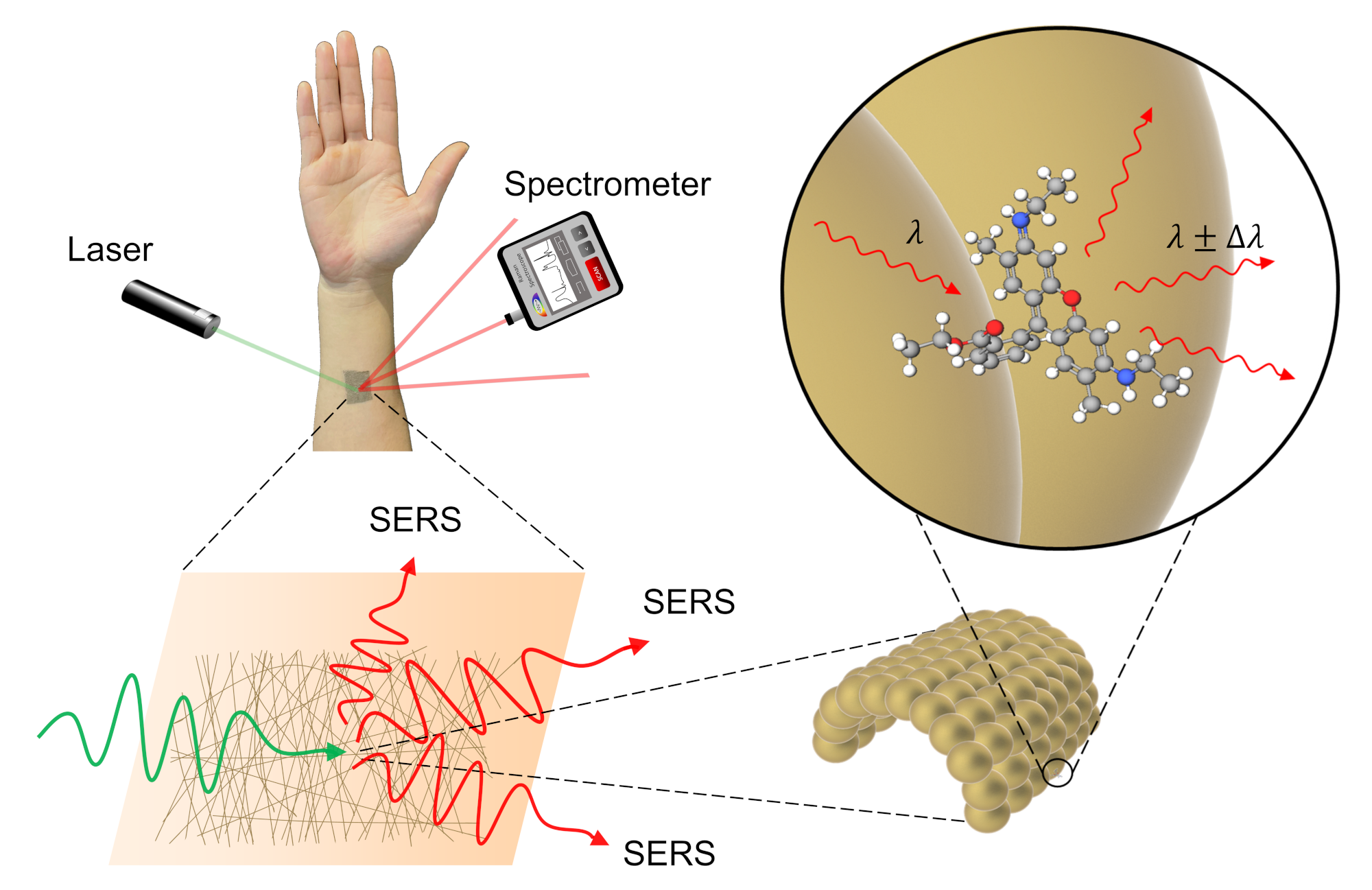
SERS in action. SERS, or surface enhanced Raman spectroscopy, is a method of detecting the presence of a chemical indirectly by using laser light and a specialized sensor. The gold mesh provides an ideal surface for taking measurements as it does not interfere with the substance being measured. ©2022 Goda et al.
Researchers created a special ultrathin sensor, spun from gold, that can be attached directly to the skin without irritation or discomfort. The sensor can measure different biomarkers or substances to perform on-body chemical analysis. It works using a technique called Raman spectroscopy, where laser light aimed at the sensor is changed slightly depending on whatever chemicals are present on the skin at that point. The sensor can be finely tuned to be extremely sensitive, and is robust enough for practical use.
Wearable technology is nothing new. Perhaps you or someone you know wears a smartwatch. Many of these can monitor certain health matters such as heart rate, but at present they cannot measure chemical signatures which could be useful for medical diagnosis. Smartwatches or more specialized medical monitors are also relatively bulky and often quite costly. Prompted by such shortfalls, a team comprising researchers from the Department of Chemistry at the University of Tokyo sought a new way to sense various health conditions and environmental matters in a noninvasive and cost-effective manner.

Stretchy sensor. Although very thin, the gold nanomesh sensor is very durable and can be stretched and deformed without breaking. Therefore, it can be adhered to many different kinds of surfaces — not just human skin — for different sensing purposes. ©2022 Goda et al.
"A few years ago, I came across a fascinating method for producing robust stretchable electronic components from another research group at the University of Tokyo," said Limei Liu, a visiting scholar at the time of the study and currently a lecturer at Yangzhou University in China. "These devices are spun from ultrafine threads coated with gold, so can be attached to the skin without issue as gold does not react with or irritate the skin in any way. As sensors, they were limited to detecting motion however, and we were looking for something that could sense chemical signatures, biomarkers and drugs. So we built upon this idea and created a noninvasive sensor that exceeded our expectations and inspired us to explore ways to improve its functionality even further."
The main component of the sensor is the fine gold mesh, as gold is unreactive, meaning that when it comes into contact with a substance the team wishes to measure — for example a potential disease biomarker present in sweat — it does not chemically alter that substance. But instead, as the gold mesh is so fine, it can provide a surprisingly large surface for that biomarker to bind to, and this is where the other components of the sensor come in.
As a low-power laser is pointed at the gold mesh, some of the laser light is absorbed and some is reflected. Of the light reflected, most has the same energy as the incoming light. However, some incoming light loses energy to the biomarker or other measurable substance, and the discrepancy in energy between reflected and incident light is unique to the substance in question. A sensor called a spectrometer can use this unique energy fingerprint to identify the substance. This method of chemical identification is known as Raman spectroscopy.
"Currently, our sensors need to be finely tuned to detect specific substances, and we wish to push both the sensitivity and specificity even further in future," said Assistant Professor Tinghui Xiao. "With this, we think applications like glucose monitoring, ideal for sufferers of diabetes, or even virus detection, might be possible."
"There is also potential for the sensor to work with other methods of chemical analysis besides Raman spectroscopy, such as electrochemical analysis, but all these ideas require a lot more investigation," said Professor Keisuke Goda. "In any case, I hope this research can lead to a new generation of low-cost biosensors that can revolutionize health monitoring and reduce the financial burden of health care."
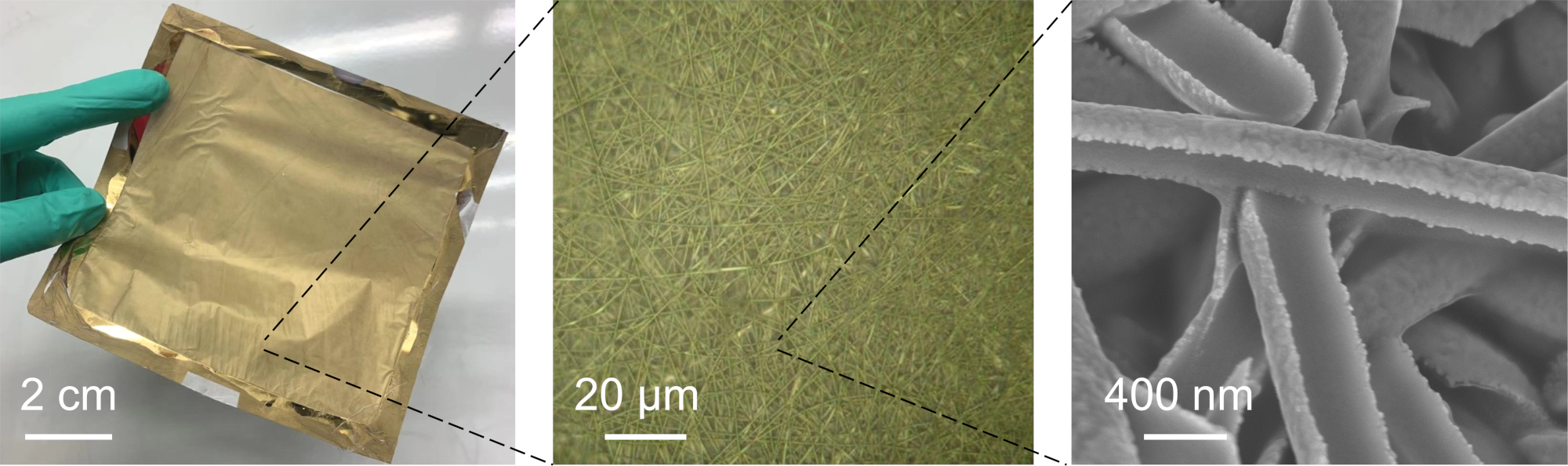
Gold mesh up close. The gold nanomesh at different magnifications. The individual fibers are around one-five-hundredth the thickness of human hair. ©2022 Goda et al.






