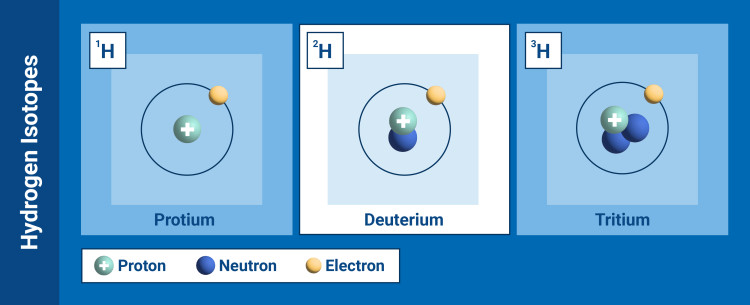
Deuterium is a stable isotope of hydrogen, which, unlike "normal" hydrogen atoms, or protium, also contains a neutron.
The isotope deuterium has one proton, one neutron and one electron.
One out of 6420 hydrogen atoms, on average, is a deuterium isotope. Deuterium isotopes are distributed in molecules that contain hydrogen, including, importantly, in all forms of water - also water in our bodies. There's 33 grams of deuterium in every cubic metre of seawater, so that the ocean contains tons of the isotope.
The natural abundance of deuterium differs from one water source to another. Water is made up of hydrogen and oxygen atoms and therefore knowledge of the deuterium abundance in a body of water provides a clue to that body's history and origin. This is a key component of isotope hydrology. Deuterium has many other uses, including in nutrition assessments and as a fuel in future fusion energy power plants. Read on, to find out more.
Tracing with the water cycle and deuterium
Deuterium can be traced throughout the water cycle, also known as the hydrological cycle: the process of water moving from the surface of the Earth to the atmosphere and back in a continuous cycle (see infographic).

The stable isotope makeup of water reflects the location of where water originated and traces its movement within the water cycle. For example, scientists can characterise both recently replenished and fossil groundwaters based on its deuterium and oxygen isotope concentrations. By having this data, scientists can evaluate the time it takes to replenish the groundwater resource and advise policymakers on how to best manage and protect the source. Read this article to learn more.
Living beings ingest water molecules which have a natural deuterium signature or "fingerprint" reflecting the water source. The isotope signature makes its way up the food web and can be found in the organic materials of different species. For example, bird feathers and claws have isotope fingerprints that can be traced back to the water source origin. By measuring the deuterium content of organic materials and correlating it to predicated rain maps, scientists can intrinsically mark the origin of an animal and trace its migration.
Tracing migration of various species using isotopic signatures of deuterium and other stable isotopes is used to create migration maps across continents. This is beneficial to support conservation efforts and protect the breeding area of animals.

Global map of animal migration (Image: CMS)
See how the migration paths of butterflies were discovered.
Using deuterium in nutrition assessments to better understand nutrition and health outcomes
Deuterium can be used in studies to improve nutrition assessments, to help understand whether weight gains or losses are due to fat or muscle, whether breastfeeding promotion campaigns are effective, and whether some population groups are at risk of high vitamin A intake. This information enables health professionals to adjust existing nutrition programmes and interventions or design new, effective ones.
A method called deuterium dilution is used to assess body composition - how much of a person's weight is fat and how much is non-fat. Water in the body contains a natural amount of deuterium. After drinking a small amount of deuterium oxide - water labelled with deuterium, the concentration of deuterium can be measured in saliva or urine and the body's ratio of fat and fat-free tissue is determined. Find more details on how the technique works here.
Deuterium can be also used to assess how much breastmilk babies consume and if babies are exclusively breastfed. Read this article for more details on how the deuterium oxide dose-to-mother technique works.
In addition, vitamin A can be labelled with deuterium to give an accurate measure of a person's vitamin A status.
Creating fusion energy with deuterium
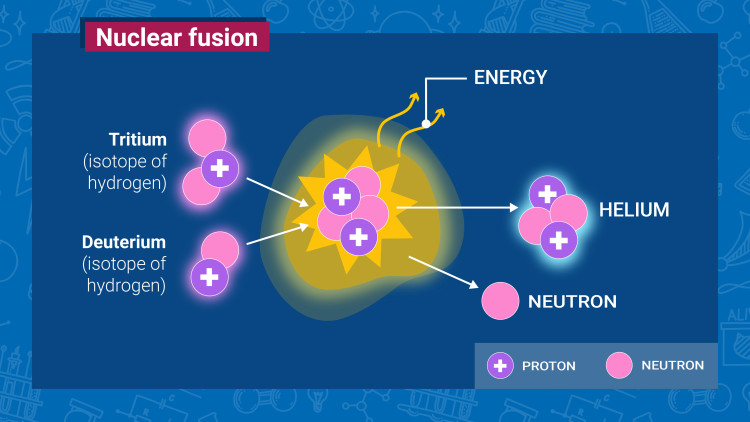
Nuclear fusion is the reaction where two light atomic nuclei combine to form one, while releasing massive amounts of energy. This process powers the stars, and it represents a long-term, sustainable, economic and safe energy option for electricity generation. Read this article






