China is a country with a high incidence of liver cancer. According to the Global Cancer Report, the number of liver cancer cases in China accounts for 46.7% of the total number of cases all over the world. Although appreciable progress in the treatment of HCC has occurred over the past few years, current therapeutic effects are far from satisfactory, and the 5‐year survival rate varies from 14% to 18%. Various kinase inhibitors, such as sorafenib and lenvatinib, come as an enormous boon to liver cancer patients, but their clinical efficacy is severely hindered owing to heterogeneity, cancer stemness and multidrug resistance of HCC.
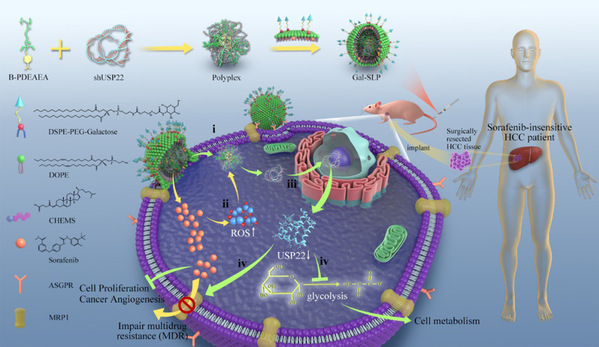
Schematic illustration of a self‐activated cascade‐responsive co‐delivery system (Gal‐SLP) for synergetic cancer therapy
To overcome drug resistance and reverse cancer stemness, the research team led by Prof. XU Xiao from the Affiliated Hangzhou First People's Hospital of the Zhejiang University School of Medicine and the research team led by Prof. SHEN Youqing from the Zhejiang University College of Chemical and Biological Engineering developed a galactose‐decorated lipopolyplex (Gal‐SLP) as an HCC‐targeting self‐activated cascade‐responsive nanoplatform to co‐delivery sorafenib and USP22 shRNA (shUSP22) for synergetic HCC therapy. Their findings were published in the journal Advanced Science.
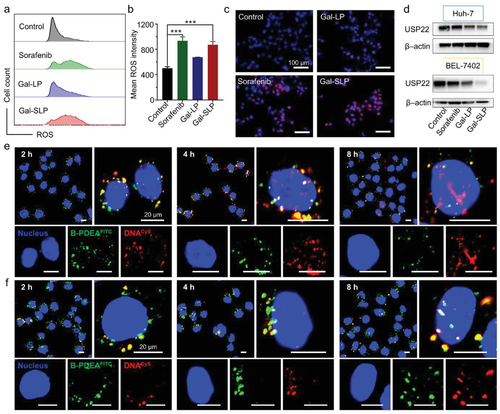
The mechanism of rapid and efficient intracellular shUSP22 release
Gal-SLPs induced an exhaustive USP22 downregulation and exhibited marked in vitro and in vivo antitumor efficiency. In particular, Gal-SLPs can induce a trio synergetic effect: i) sorafenib elevated intracellular levels of reactive oxygen species (ROS), which oxidize B-PDEAEA to trigger rapid shUSP22 release for efficient gene downregulation; ii) the downregulation of USP22 led to downregulation of multidrug resistance-associated protein 1 (MRP1) and inhibition of glycolysis, dramatically impairing MDR and achieving higher intracellular sorafenib accumulation, thus generating an ROS-responsive positive feedback loop; iii) the downregulation of USP22 suppressed the cell metabolism of cancer cells and further influenced cancer stemness.
Furthermore, a sorafenib‐insensitive patient‐derived xenograft (PDX) model was established to evaluate in vivo antitumor effect of Gal‐SLPs. Gal-SLPs exhibited much better antitumor efficiency, strongly suppressing the tumor growth during the whole experimental period, resulting in a tumor-growth inhibition rate (TIR %) of 82.7 ± 4.9% in terms of tumor weight at the end of experiment, significantly higher than that of sorafenib (53.4 ± 15.5%) and Gal-LPs (55.0 ± 7.8%). And biosafety evaluation assays supported the excellent safety profile of Gal-SLPs. Therefore, Gal-SLPs are expected to have great potential in the clinical treatment of HCC.
There is still a long way for human to surmount cancer, but Prof. XU Xiao and his team believed that the development of precision medicine and multidisciplinary cooperation would bring a new insight into cancer therapy. "Combination and precision cancer therapy is undoubtedly the direction of future development," XU Xiao said. And the research findings have witnessed a successful cooperation of medicine, chemistry and biology and given hope to surmounting cancer.






